2024 AACR:中国双抗ADC们卷起来了?
近期,CDE网站显示BL-B01D1拟纳入突破性疗法,用于二线及以上治疗复发性或转移性鼻咽癌患者,有望成为首款获批上市的双抗ADC。
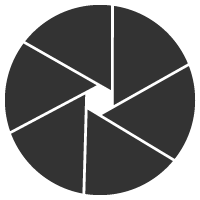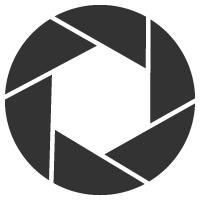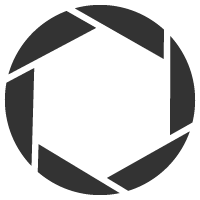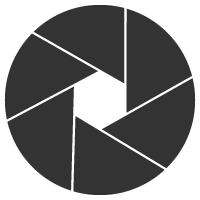
这些消息也引爆了双抗ADC的研发热情,目前全球尚无双抗ADC获批上市,但是其研发如火如荼,各大biotech纷纷入局。本文盘点下今年AACR上公开的双抗ADC最新进展(国内篇)。
01

02
VBC101-F11(EGFR/cMet)

VBC103(Trop2/Nectin4)

03

04

05


06



07

08

09
写在最后

 主要参考文献
主要参考文献
1.Abstract LB055: IBI3001: A potentially first-in-class site-specifically conjugated B7-H3/EGFR bispecific ADC for multiple solid tumors
2.Abstract LB043: VBC101-F11: An innovative EGFR/cMet bispecific antibody drug conjugate (ADC) targeting key oncogenic drivers in solid tumors
3.Abstract LB448: VBC103: An innovative Trop2/Nectin4 targeted bispecific antibody drug conjugate (ADC) in bladder urothelial carcinoma (UC), triple-negative breast cancer (TNBC) and beyond
4.Abstract CT179: Safety and efficacy of JSKN003 in patients with advanced/metastatic solid tumors: A first-in-human, dose-escalation, multicenter, open-label, phase I study
5.Abstract 6580: A Novel EGFR x cMET bispecific ADC PRO1286 demonstrated broad antitumor activity and promising tolerability in preclinical models
6.Abstract 1882: DXC024, a novel anti-TROP2/EGFR bispecific antibody and tubulysin conjugate, for targeted treatment of highly TROP2- or EGFR-expressing tumors
7.Abstract 3147: DXC025, a novel anti-MUC1/EGFR bispecific antibody-tubulysin conjugate with a function linker, exhibits potent anti-tumor efficacy
8.Abstract 2620: BCG016, a first-in-class bispecific antibody-drug conjugate targeting 5T4 and MUC1, demonstrates potent preclinical anti-tumor activity
9.Abstract 2618: BCG017, a bispecific ADC targeting PTK7 and EGFR exhibits anti-tumor efficacy in PDX models
10.Abstract 2619: A novel EGFR × HER3-targeting bispecific antibody drug-conjugate, BCG019, demonstrates robust anti-tumor efficacy in preclinical evaluation
11.Abstract 5083: Discovery of an asymmetric IgG-like bispecific ADC targeting EGFR/HER3
12.Abstract 1871: Discovery and characterization of NXV01c, an EGFR × cMET bispecific nanobody drug conjugate with potent anti-tumor activity
13.Abstract 2621: Preclinical Efficacy of DM002, a bispecific HER3 × MUC1 antibody-drug conjugate with a novel DNA topoisomerase I inhibitor, in solid tumor models
撰稿人 | BiG专栏 BiG生物创新社
责任编辑 | 邵丽竹
审核人 | 何发
邵丽竹
何发
热点文章
-
一文看懂新药研发到上市的全流程
2025-11-25
-
《药包材GMP(2025)》与药品GMP深度对比:核心差异、新增要点与实施指引
2025-11-25
-
浅谈非最终灭菌产品制剂车间无菌工艺模拟试验中的要点与设计
2025-11-18
-
人工智能在生物医药领域的应用前景分析及对策建议
2025-11-14
-
制药生产标准不断提升:原料药与高活性原料药的密闭隔离解决方案
2025-11-19
-
一文掌握一条产业链:医药板块
2025-11-17
-
从"制造"到"智造",默克如何进行数字化转型?
2025-11-21
-
基于CFD仿真技术的灌装机充氮装置设计优化
本文以某制药产线的灌装机设备为研究对象,采用计算流体动力学(CFD)仿真技术对充氮装置的充氮性能进行分析,并结合分析结果对氮幕结构进行了优化设计。随后,针对优化方案进行性能仿真验证,结果显示优化后的顶空残氧量降低至0.252%。为了进一步验证优化方案的实际效果,将优化方案应用于实际产线进行性能测试,测得的顶空残氧量为0.68%,这一结果满足了小于1%的要求,表明其充氮保护性能已达到国际先进水平。
作者:王志刚、刘依宽、刘佳鑫
-
药品密封性检测 :用户需求与优化
-
可控冻融系统在生物原液上的应用
-
人用疫苗生产数字化转型
-
药包材生产质量管理的进阶策略
-
药厂洁净区域风量和压差的控制策略













评论
加载更多