我国化学仿制药参比制剂落选品种的特征分析
Part
1
1.1
参比制剂遴选、发布政策沿革
1.2
已公示参比制剂品种
Part
2
2.1
落选品种的公示批次
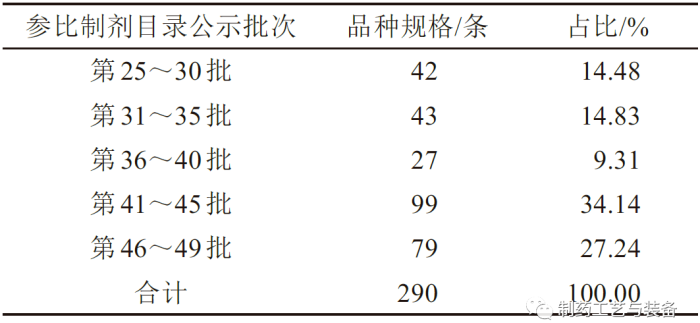
表1 落选化学仿制药参比制剂的批次及品种规格
2.2
落选品种未通过审评的原因
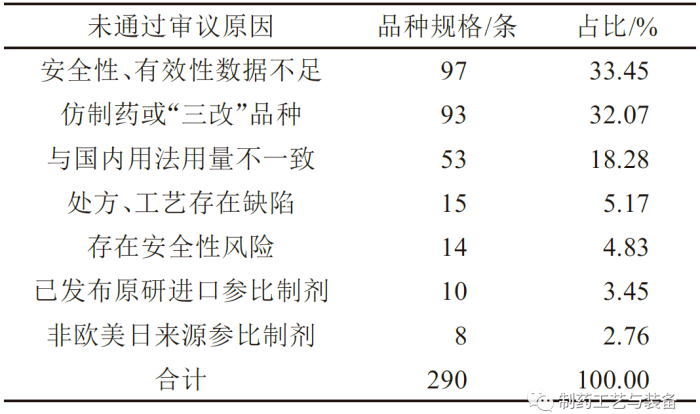
表2 落选化学仿制药参比制剂的落选原因
2.3
落选品种的剂型
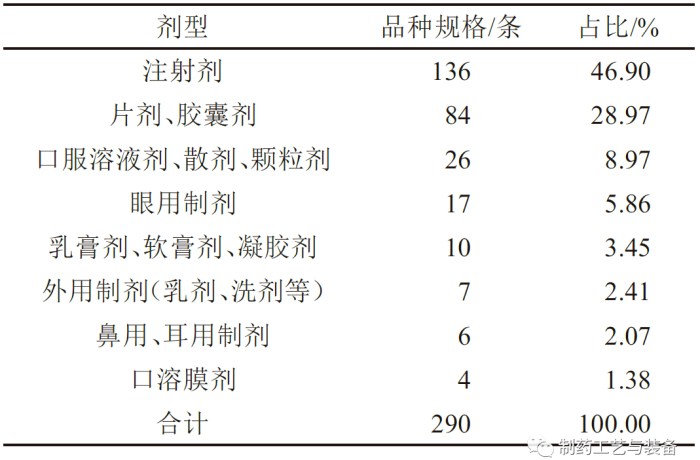
表3 落选化学仿制药参比制剂的剂型
Part
3
3.1
避免安全性、有效性数据不足
3.2
仿制药或“三改”品种参比制剂的注意事项
3.3
参比制剂用法用量、处方及工艺
3.4
参比制剂的来源
Part
4
参考文献
[1] 国家食品药品监督管理局. 关于发布化学仿制药参比制剂遴选与确定程序的公告[EB/OL]. (2019-03-25)[2021-10-06]. https://www. nmpa. gov. cn/xxgk/ggtg/qtggtg/20190328162401710.html.National Medical Produces Administration (NMPA).Announcement on the selection and determination procedure of reference preparations for chemical generic drugs [EB/OL]. (2019-03-25) [2021-10-06]. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20190328162401710.html.
[2] 刘冬, 哈莉莉, 李芳, 等. 仿制药质量和疗效一致性评价参比制剂申请平台情况分析及参比制剂选择的几点考虑[J]. 中国临床药理学杂志, 2019, 35(23): 3158-3161.Liu D, Ha L L, Li F, et al. Analysis and consideration on the applications of the reference preparation platform for the chemical generic drugs [J]. Chin J Clin Pharmacol,2019, 35(23): 3158-3161.
[3] 胡宇, 宗欣, 于淼, 等. 国外仿制药一致性评价经验与启示[J]. 中国药物评价, 2020, 37(5): 327-331.Hu Y, Zong X, Yu N, et al. Experience and enlightenment of foreign generic drug consistency evaluation [J]. Chin J Drug Eval, 2019, 35(23): 3158-3161.
[4] 许鸣镝, 牛剑钊, 杨东升, 等. 欧盟仿制药参比制剂介绍[J]. 中国新药杂志, 2017, 26(24): 2933-2936.Xu M D, Niu J Z, Yang D S, et al. The introduction of the reference medicinal products for marketing authorization application of generic medicinal products in the EU [J].Chin J New Drugs, 2017, 26(24): 2933-2936.
[5] 国务院. 国务院关于改革药品医疗器械审评审批制度的意见[EB/OL]. (2015-08-09)[2021-10-06].http://www.gov.cn/zhengce/content/2015-08/18/content_10101.htm.The State Council. Opinions of the State Council on reforming the review and approval system of drugs and medical devices [EB/OL]. (2015-08-09) [2021-10-06].http://www. gov. cn/zhengce/content/2015-08/18/content_10101.htm.
[6] 国务院办公厅. 国务院办公厅关于开展仿制药质量和疗效一致性评价的意见[EB/OL]. (2016-03-05) [2021-10-06]. http://www. gov. cn/zhengce/content/2016-03/05/content_5049364.htm.
General Office of the State Council. Opinions of General Office of the State Council on the consistency evaluation of quality and efficacy of generic drugs [EB/OL]. (2016-03-05) [2021-10-06]. http://www. gov. cn/zhengce/content/2016-03/05/content_5049364.htm.
[7] 国家食品药品监督管理总局. 关于发布仿制药质量和疗效一致性评价参比制剂备案与推荐程序的公告[EB/OL]. (2016-05-19) [2021-10-06]. https://www. nmpa. gov.cn/xxgk/ggtg/qtggtg/20160519194501890.html.
State Food and Drug Administration. Announcement on filing and recommending procedures for reference preparations for consistency evaluation of quality and efficacy of generic drugs [EB/OL]. (2016-05-19) [2021-10-06]. https://www. nmpa. gov. cn/xxgk/ggtg/qtggtg/20160519194501890.html.
[8] 国家药品监督管理局药品审评中心. 关于开通仿制药质量和疗效一致性评价专栏的通知[EB/OL]. (2017-12-08) [2021-10-06]. https://www. cde. org. cn/yzxpj/news/viewInfoCommon/2fd4e28bb88650f95530049ef3291eef.Center for Drug Evaluation, National Medical Produces Administration. Notice on opening the column of quality and efficacy consistency evaluation of generic drugs [EB/OL]. (2017-12-08) [2021-10-06]. https://www.cde.org.cn/yzxpj/news/viewInfoCommon/2fd4e28bb88650f95530049ef3291eef.
[9] 国家药品监督管理局药品审评中心. 关于公开征求«化学仿制药参比制剂遴选申请资料要求(征求意见稿)»意见的通知[EB/OL]. (2020-08-05) [2021-10-06]. https://www. cde. org. cn/yzxpj/news/viewInfoCommon/c9bf07594d518cdfdbca3daeb4f3a189.
Center for Drug Evaluation, National Medical Produces Administration. Notice on soliciting comments on application requirements for selection of reference preparations for chemical generic drugs (public draft)[EB/OL]. (2020-08-05) [2021-10-06]. https://www. cde.org.cn/yzxpj/news/viewInfoCommon/c9bf07594d518cdfdbca3daeb4f3a189.
[10] 国家药品监督管理局药品审评中心. 关于发布«化学仿制药参比制剂遴选申请资料要求»的通告[EB/OL].(2020-10-19)[2021-10-06].https://www.cde.org.cn/yzxpj/news/viewInfoCommon/1c15428178255464bd316f8c171a038c.
Center for Drug Evaluation, National Medical Produces Administration. Circular on the issuance of application requirements for selection of reference preparations for chemical generic drugs [EB/OL]. (2020-10-19) [2021-10-06]. https://www. cde. org. cn/yzxpj/news/viewInfo Common/1c15428178255464bd316f8c171a038c.
[11] 国家食品药品监督管理总局. 总局关于发布化学药品注册分类改革工作方案的公告[EB/OL]. (2016-03-04)[2021-10-08]. http://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20160309151801706.html.
State Food and Drug Administration (SFDA).Announcement on the work plan for classification reform of chemical drugs registration [EB/OL]. (2016-03-04)[2021-10-08].http://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20160309151801706.html.
[12] 国家药品监督管理局. 国家药监局关于发布化学药品注册分类及申报资料要求的通告[EB/OL]. (2020-06-30)[2021-10-10]. https://www. nmpa. gov. cn/zhuanti/ypzhcglbf/ypzhcglbfzhcwj/20200630180301525.html.National Medical Produces Administration (NMPA).Circular on the issuance of registration classification and application requirements for chemical drugs [EB/OL].(2020-06-30) [2021-10-10]. https://www. nmpa. gov. cn/zhuanti/ypzhcglbf/ypzhcglbfzhcwj/20200630180301525.html.
[13] 刘冬, 李芳, 安娜, 等. 我国化学仿制药参比制剂遴选进展及其几点考虑[J]. 中国临床药理学杂志, 2021,37(6): 783-787.Liu D, Li F, An N, et al. Progress and consideration of reference selection of chemical generic drugs in China[J]. Chin J Clin Pharmacol, 2021, 37(6): 783-787.
[14] 国家药品监督管理局药品审评中心. 国家药监局药审中心关于发布«化学药品注册受理审查指南(试行)»的通告[EB/OL]. (2020-07-03) [2020-10-06]. https://www.cde. org. cn/main/news/viewInfoCommon/6386d7ca5a1515db4259acb1b8f16333.
Center for Drug Evaluation, National Medical Produces Administration. Circular on the issuance of guidelines for accepting and reviewing registration of chemical drugs(trial) [EB/OL]. (2020-07-03) [2021-10-06]. https://www.cde. org. cn/main/news/viewInfoCommon/6386d7ca5a1515db4259acb1b8f16333.
[15] 国家食品药品监督管理总局. 总局关于仿制药质量与疗效一致性评价中改规格药品(口服固体制剂)评价一般考虑等3个技术指南的通告[EB/OL]. (2017-02-17)[2021-10-10]. https://www. nmpa. gov. cn/directory/web/nmpa/xxgk/ggtg/qtggtg/20170217105901435.html.
State Food and Drug Administration (SFDA). Notice of 3 technical guidelines on general considerations for evaluation of modified drugs (oral solid preparations) in evaluation of quality and efficacy consistency of generic drugs [EB/OL]. (2017-02-17) [2021-10-10]. https://www.nmpa. gov. cn/directory/web/nmpa/xxgk/ggtg/qtggtg/20170217105901435.html.
[16] 国家药品监督管理局药品审评中心. 国家药监局药审中心关于发布«化学仿制药参比制剂目录(第四十六批)»的公示[EB/OL]. (2020-06-29)[2021-10-08]. https://www. cde. org.cn/yzxpj/news/viewInfoCommon/d29726ca5441451a55f42ff247ccf93b.
Center for Drug Evaluation, National Medical Produces Administration. Publication of catalogue of reference preparations for chemical generic drugs (46th batch) [EB/OL]. (2020-06-29) [2021-10-08]. https://www. cde. org. cn/yzxpj/news/viewInfoCommon/d29726ca5441451a55f42ff247ccf93b.
[17] 国家药品监督管理局药品审评中心. 常见一般技术问题(序号2) [EB/OL]. (2021-06-08) [2021-10-08]. https://www. cde.org. cn/main/xxgk/listpage/07edef25f1e7354bfd8490baa0ce056b.
Center for Drug Evaluation, National Medical Produces Administration. Common technical questions (No 2.)[EB/OL]. (2021-06-08) [2021-10-08]. https://www. cde.org. cn/main/xxgk/listpage/07edef25f1e7354bfd8490baa0ce056b.
[18] 赵飞, 赵紫楠, 薛薇, 等. 我国国家基本药物目录中仿制药参比制剂设立情况研究[J]. 中国药事, 2021, 35(9): 1000-1006.Zhao F, Zhao Z N, Xue W, et al. On the Establishment of Generic Reference Formulations in China's National Essential Drug List [J]. Chin Pharma Aff, 2021, 35(9):1000-1006.
撰稿人 | 刘冬、韩鸿璨、安娜、李芳、王骏
责任编辑 | 胡静
审核人 | 何发
邵丽竹
何发
热点文章
-
一文看懂新药研发到上市的全流程
2025-11-25
-
《药包材GMP(2025)》与药品GMP深度对比:核心差异、新增要点与实施指引
2025-11-25
-
浅谈非最终灭菌产品制剂车间无菌工艺模拟试验中的要点与设计
2025-11-18
-
制药生产标准不断提升:原料药与高活性原料药的密闭隔离解决方案
2025-11-19
-
一文掌握一条产业链:医药板块
2025-11-17
-
从"制造"到"智造",默克如何进行数字化转型?
2025-11-21
-
拜耳这波数字化操作,直接解放全自动片剂生产车间劳动力!
2025-11-18
-
基于CFD仿真技术的灌装机充氮装置设计优化
本文以某制药产线的灌装机设备为研究对象,采用计算流体动力学(CFD)仿真技术对充氮装置的充氮性能进行分析,并结合分析结果对氮幕结构进行了优化设计。随后,针对优化方案进行性能仿真验证,结果显示优化后的顶空残氧量降低至0.252%。为了进一步验证优化方案的实际效果,将优化方案应用于实际产线进行性能测试,测得的顶空残氧量为0.68%,这一结果满足了小于1%的要求,表明其充氮保护性能已达到国际先进水平。
作者:王志刚、刘依宽、刘佳鑫
-
药品密封性检测 :用户需求与优化
-
可控冻融系统在生物原液上的应用
-
人用疫苗生产数字化转型
-
药包材生产质量管理的进阶策略
-
药厂洁净区域风量和压差的控制策略













评论
加载更多