用于胶囊的粉末微量灌装技术
Part
1
1.1
实验设备
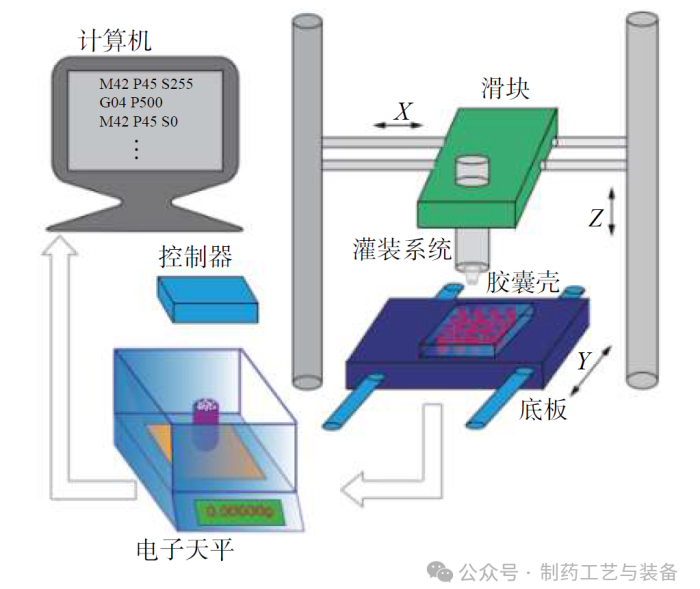
图1 振动驱动粉末灌装系统示意图
1.2
实验材料
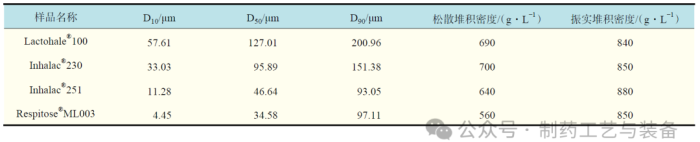
表1 吸入级乳糖样品物性
1.3
样品制备与处理
1.4
灌装剂量分析
Part
2
2.1
流量控制机理
2.2
灌装剂量均一性
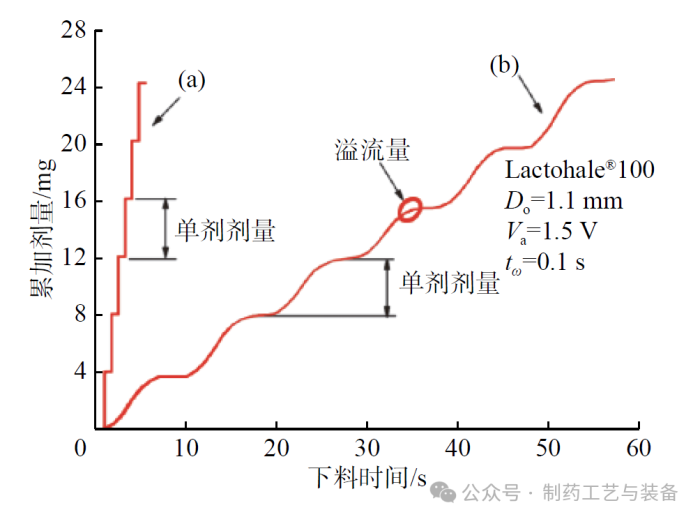
图2 累加剂量与下料时间的关系
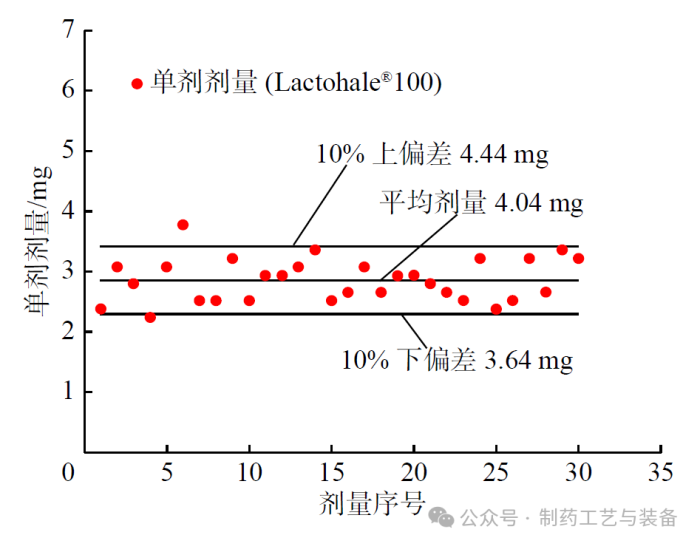
图3 Lactohale®100 单剂剂量分布
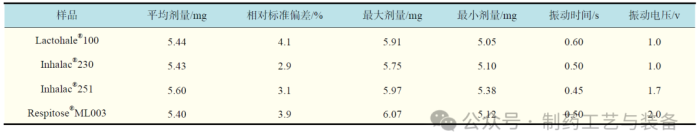
表2 不同样品目标剂量为5.5 mg的灌装结果
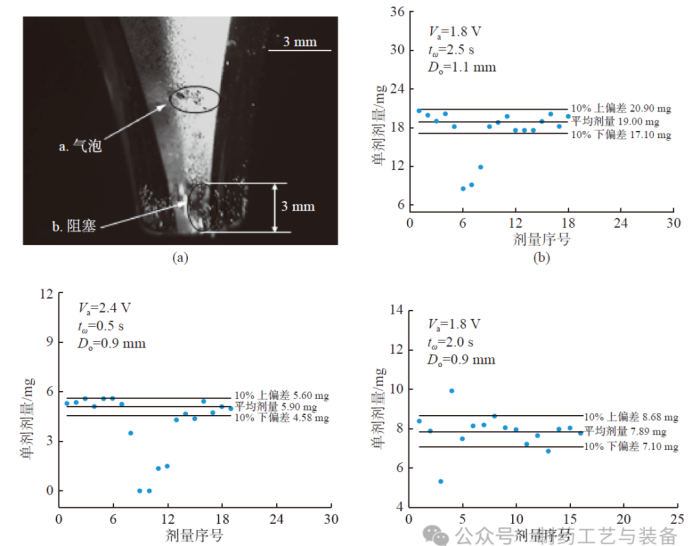
图4 气泡和阻塞现象对Respitose®ML003 单剂剂量分布的影响
2.3
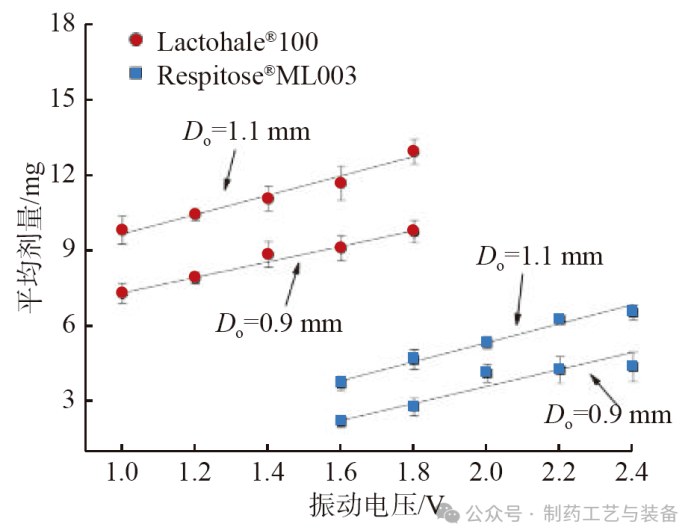
振动电压对粉体平均灌装剂量的影响
2.4
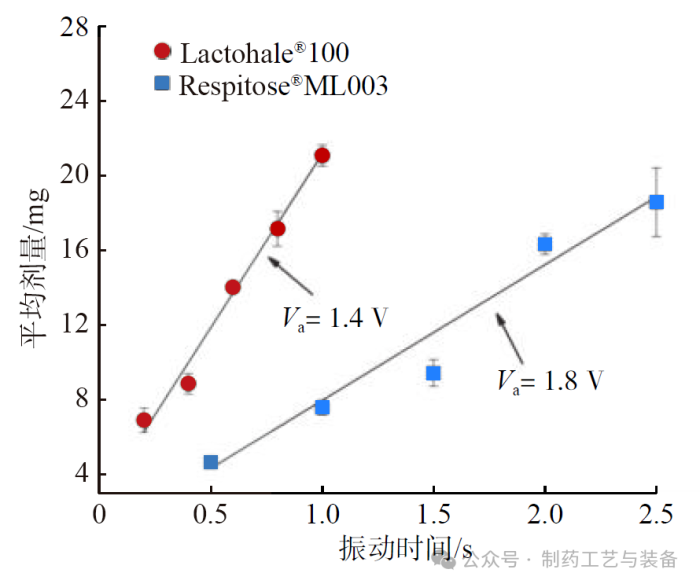
振动时间对粉体平均灌装剂量的影响
Part
3
参考文献
[1]FAULHAMMER E, LLUSA M, RADEKE C, et al. The effects of material attributes on capsule fill weight and weight variability in dosator nozzle machines[J].International Journal of Pharmaceutics, 2014, 47(1/2):332–338.
[2]LLUSA M, FAULHAMMER E, BISERNI S, et al. The effects of powder compressibility, speed of capsule filling and pre-compression on plug densification[J]. International Journal of Pharmaceutics, 2014, 471(1/2): 182–188.
[3]EDWARDS D. Applications of capsule dosing techniques for use in dry powder inhalers[J]. Therapeutic Delivery,2010, 1(1): 195–201.
[4]STEGEMANN S, KOPP S, BORCHARD G, et al.Developing and advancing dry powder inhalation towards enhanced therapeutics[J]. European Journal of Pharmaceutical Sciences, 2013, 48(1/2): 181–194.
[5]DANIHER D I, ZHU J. Dry powder platform for pulmonary drug delivery[J]. Particuology, 2008, 6(4):225–238.
[6]PODCZECK F. The development of an instrumented tampfilling capsule machine I: Instrumentation of a Bosch GKF 400S machine and feasibility study[J]. European Journal of Pharmaceutical Sciences, 2000, 10(4): 267–274.
[7]PODCZECK F, NEWTON J M. Powder filling into hard gelatine capsules on a tamp filling machine[J].International Journal of Pharmaceutics, 1999, 185(2):237–254.
[8]NEWTON J M. Filling hard gelatin capsules by the dosator nozzle system--is it possible to predict where the powder goes? [J]. International Journal of Pharmaceutics, 2012,425(1/2): 73–74.
[9]LU X, YANG S, ChEN L, et al. Dry powder microfeeding system for solid freeform fabrication[C]//International Solid Freeform Fabrication Symposium, 2006: 636-643.
[10]BESENHARD M O, KARKALA S K, FAULHAMMER E, et al. Continuous feeding of low-dose APIs via periodic micro dosing[J]. International Journal of Pharmaceutics,2016, 509(1/2): 123–134.
[11]LI Z Q, YANG S F. Nanobiomaterials library synthesis for high-throughput screening using a dry powder printing method[J]. Nano Life, 2012, 2(1): 1250006.
[12]MATSUSAKA S, URAKAWA M, MASUDA H. Microfeeding of fine powders using a capillary tube with ultrasonic vibration[J]. Advanced Powder Technology,1995, 6(4): 283–293.
[13]LU X S, YANG S F, EVANS J R G. Studies on ultrasonic microfeeding of fine powders[J]. Journal of Physics D:
[14]Applied Physics, 2006, 39(11): 2444–2453.BESENHARD M O, FAULHAMMER E, FATHOLLAHI S, et al. Accuracy of micro powder dosing via a vibratory sieve-chute system[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2015, 94: 264–272.
[15]CHEN X L, SEYFANG K, STECKEL H. Development of a micro dosing system for fine powder using a vibrating capillary. Part 1: the investigation of factors influencing on the dosing performance[J]. International Journal of Pharmaceutics, 2012, 433(1/2): 34–41.
[16]CHEN X L, SEYFANG K, STECKEL H. Development of a micro-dosing system for fine powder using a vibrating capillary. Part 2: the implementation of a process analytical technology tool in a closed-loop dosing system[J].International Journal of Pharmaceutics, 2012, 433(1/2):42–50.
[17]SCHULZE D. Powders and bulk solids: Behavior,characterization, storage and flow[M]. Berlin: SpringerVerlag, 2008.
[18]FAULHAMMER E, FINK M, LLUSA M, et al. Low-dose capsule filling of inhalation products: Critical material attributes and process parameters[J]. International Journal of Pharmaceutics, 2014, 473(1/2): 617–626.
[19]HENDRICKS C D. Charging macroscopic particles[M]//MOORE A D. Electrostatics and Its Applications. New York: John Wiley and Sons, 1973.
[20]FENG J Q, HAYS D A. Relative importance of electrostatic forces on powder particles[J]. Powder Technolog, 2003, 135/136: 65–75.
[21]MATSUSAKA S, KOBAYAKAWA M, MIZUTANI M, et al. Bubbling behavior of a fluidized bed of fine particles caused by vibration-induced air inflow[J]. Scientific Reports, 2013, 3: 1190.
撰稿人 | 刘环、陈岚、李宗齐、陈东浩 上海理工大学学报
责任编辑 | 胡静
审核人 | 何发
邵丽竹
何发
热点文章
-
PDE值的计算
2025-04-15
-
批生产记录如何管理,才能符合GMP要求?
2025-04-24
-
OEB4级药品生产的法规要求
2025-05-16
-
抗体-药物偶联物的偶联技术总结
2025-05-11
-
发酵工业中常用的10种菌简介
2025-04-29
-
抗体原液冻融过程中稳定性探究及冻融工艺建立
2025-04-28
-
2024年生物制药行业收入Top20
2025-05-22
-
基于CFD仿真技术的灌装机充氮装置设计优化
本文以某制药产线的灌装机设备为研究对象,采用计算流体动力学(CFD)仿真技术对充氮装置的充氮性能进行分析,并结合分析结果对氮幕结构进行了优化设计。随后,针对优化方案进行性能仿真验证,结果显示优化后的顶空残氧量降低至0.252%。为了进一步验证优化方案的实际效果,将优化方案应用于实际产线进行性能测试,测得的顶空残氧量为0.68%,这一结果满足了小于1%的要求,表明其充氮保护性能已达到国际先进水平。
作者:王志刚、刘依宽、刘佳鑫
-
药品密封性检测 :用户需求与优化
-
可控冻融系统在生物原液上的应用
-
人用疫苗生产数字化转型
-
药包材生产质量管理的进阶策略
-
药厂洁净区域风量和压差的控制策略














评论 0
正在获取数据......