欧盟与我国GMP无菌药品附录差异分析与探讨(后附欧盟GMP无菌指南中英对照术语表)
Part
1
整体性差异

表1 欧盟与我国GMP无菌药品附录整体性差异对比
Part
2
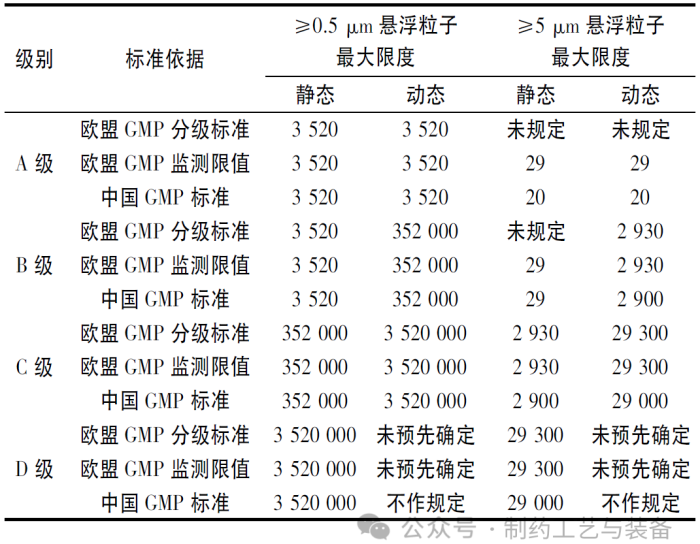
标准对比(单位: 每立方米)
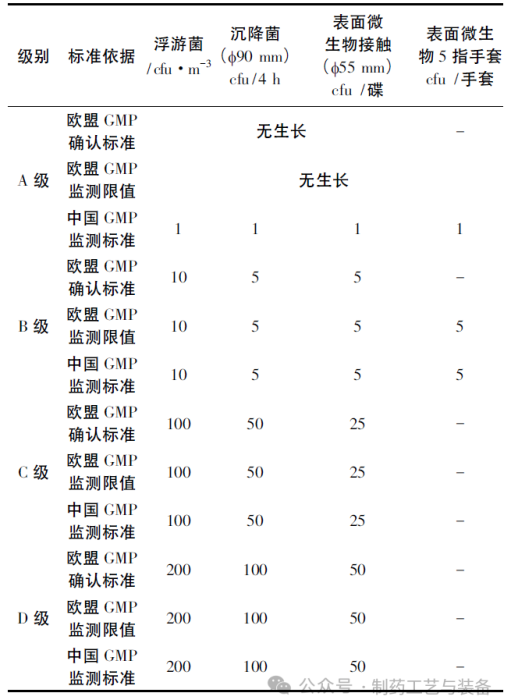
表3 欧盟与中国GMP各级别环境微生物标准对比
Part
3
参考文献
[1] EU.Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use,Annex1: Manufacture of Sterile Medicinal Products[EB/OL].[2022- 08 - 25]. https: / /health. ec. europa. eu /system/files /2022-08 /20220825_gmp-an1_en_0.pdf
[2] EU.Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use,Annex1: Manufacture of Sterile Medicinal Products (corrected version) [EB/OL].[2008 - 12 - 15]. https: / /health. ec.europa.eu /system/files /2016-11 /2008_11_25_gmp-an1_en_0.pdf.
[3] 国家食品药品监督管理局.关于发布《药品生产质量管理规范( 2010 年修订) 》无菌药品等5个附录的公告[EB/OL].[2011 - 02 - 24]. https: / /www. nmpa. gov.cn /xxgk /ggtg /qtggtg /20110224164501312.html
[4] 郑金旺.2020 版欧盟GMP 附录1 草案的主要变化解读及对国内无菌产品生产的影响分析[J].化工与医药工程,2020,41(2) : 65-70.
[5] 曹鸿雁,韩莹,胡敬峰.山东省无菌制剂生产质量风险分析与探讨[J].中国药事,2018,32(7) : 901-905.
[6] 国家食品药品监督管理局药品认证管理中心. 药品GMP 指南: 无菌药品[M]. 北京: 中国医药科技出版社,2011: 118-123.
[7] 任杏珠.医药洁净厂房空调系统确认和环境监测取样点选取探讨[J].煤炭与化工,2022,45(1) : 144-150.
[8] 国家药品监督管理局.国家药品监督管理局关于发布除菌过滤技术及应用指南等3个指南的通告[EB/OL].[2018 - 09 - 11]. https: / /www. nmpa. gov. cn /ylqx /ylqxggtg /ylqxqtgg /20180911170301439.html.
[9] 胡敬峰,许丹,韩莹.除菌过滤技术在药品生产应用中存在的问题与对策[J].中国药事,2020,34(12) : 1-5.
[10] PIC/S.Revised Annex 1 Manufacture of Sterile Medicinal Products[EB/OL].[2022- 09- 09]. https: / /picscheme.org /docview/4737.
[11] 国家药品监督管理局.国家药监局研究推进加入PIC/S工作[EB/OL].[2022 - 06 - 29]. https: / /www. nmpa.gov.cn /yaopin /ypjgdt /20220629093952150.html.
Critical zone – A location within the aseptic processing area in which product and critical surfaces are exposed to the environment.
关键区:位于无菌工艺区内的、产品和关键表面暴露于其中的位置。
Critical intervention – An intervention (corrective or inherent) into the critical zone.
关键干预:对关键区的干预 (纠正性或固有性干预)。
D-value – The value of a parameter of sterilisation (duration or absorbed dose) required to reduce the number of viable organisms to 10 per cent of the original number.
D 值:将活性微生物数量减少至原始数量的 10%的灭菌参数值 (持续时间或吸收剂量)。
Dead leg – Length of non-circulating pipe (where fluid may remain static) that is greater than 3 internal pipe diameters.
死角:长度大于管道内径 3 倍的非循环管 (流体在此处可能保持静止)。
Decommission – When a process, equipment or cleanroom are closed and they will not be used again.
停用:工艺、设备或洁净室关闭并且将不再使用。
Decontamination – The overall process of removal or reduction of any contaminants (chemical, waste,residue or microorganisms) from an area, object, or person. The method of decontamination used (e.g.cleaning, disinfection, sterilisation) should be chosen and validated to achieve a level of cleanliness appropriate to the intended use of the item decontaminated. See also Bio-decontamination.
净化:消除或减少区域、物体或人体的任何污染物 (化学物质,) 废物,残留物或微生物) 的综合过程。所用净化方法 (例如清洁,消毒,灭菌) 应进行选择和验证,以达到适用于被净化物品预期用途的洁净水平另请参见生物净化。
Depyrogenation – A process designed to remove or inactivate pyrogenic material (e.g. endotoxin) to a specified minimum quantity.
除热原:旨在将致热物质 (例如内毒素) 去除或灭活至规定最小量的过程。
Disinfection – The process by which the reduction of the number of microorganisms is achieved by the irreversible action of a product on their structure or metabolism, to a level deemed to be appropriate for a defined purpose.
消毒:通过产品结构或代谢的不可逆作用,将微生物数量减少至被认为适合某特定用途的水平的过程。
Endotoxin – A pyrogenic product (i.e. lipopolysaccharide) present in the Gram negative bacterial cell wall. Endotoxin can lead to reactions in patients receiving injections ranging from fever to death.
内毒素:革兰氏阴性细菌细胞壁中存在的致热产物(即脂多糖)。内毒素能导致接受注射的患者发热至死亡的反应。
Equilibration time – Period which elapses between the attainment of the sterilisation temperature at the reference measurement point and the attainment of the sterilisation temperature at all points within the load.
平衡时间:从参考测量点达到灭菌温度到负载内所有点达到灭菌温度之间的时间。
Extractables - Chemical entities that migrate from the surface of the process equipment, exposed to an appropriate solvent at extreme conditions, into the product or material being processed.
溶出物:当在极端条件下暴露于适当溶剂中,从工艺设备表面迁移至被加工的产品或物料中的化学实体。
First Air – Refers to filtered air that has not been interrupted prior to contacting exposed product and product contact surfaces with the potential to add contamination to the air prior to reaching the critical zone.
初始气流:指在接触暴露的产品和产品接触表面之前没有被阻碍从而在到达关键区之前不太可能被污染的经过过滤的气流。
Filter Integrity test - A test to confirm that a filter (product, gas or HVAC filter) retain their retentive properties and have not been damaged during handling, installation or processing.
过滤器完整性测试:一种用以确认过滤器(产品,气体或 HVAC 过滤器)保持其截留特性并且在处理、安装或加工过程中没有被损坏的测试。
撰稿人 | 胡敬峰、明奕、王金子、宋凯、樊红延、冯巧巧 药学研究
责任编辑 | 胡静
审核人 | 何发
邵丽竹
何发
热点文章
-
一文看懂新药研发到上市的全流程
2025-11-25
-
《药包材GMP(2025)》与药品GMP深度对比:核心差异、新增要点与实施指引
2025-11-25
-
浅谈非最终灭菌产品制剂车间无菌工艺模拟试验中的要点与设计
2025-11-18
-
制药生产标准不断提升:原料药与高活性原料药的密闭隔离解决方案
2025-11-19
-
一文掌握一条产业链:医药板块
2025-11-17
-
从"制造"到"智造",默克如何进行数字化转型?
2025-11-21
-
拜耳这波数字化操作,直接解放全自动片剂生产车间劳动力!
2025-11-18
-
基于CFD仿真技术的灌装机充氮装置设计优化
本文以某制药产线的灌装机设备为研究对象,采用计算流体动力学(CFD)仿真技术对充氮装置的充氮性能进行分析,并结合分析结果对氮幕结构进行了优化设计。随后,针对优化方案进行性能仿真验证,结果显示优化后的顶空残氧量降低至0.252%。为了进一步验证优化方案的实际效果,将优化方案应用于实际产线进行性能测试,测得的顶空残氧量为0.68%,这一结果满足了小于1%的要求,表明其充氮保护性能已达到国际先进水平。
作者:王志刚、刘依宽、刘佳鑫
-
药品密封性检测 :用户需求与优化
-
可控冻融系统在生物原液上的应用
-
人用疫苗生产数字化转型
-
药包材生产质量管理的进阶策略
-
药厂洁净区域风量和压差的控制策略













评论
加载更多