Current Federal law requires that a drug be the subject of an approved marketing application before it is transported or distributed across state lines. Because a sponsor will probably want to ship the investigational drug to clinical investigators in many states, it must seek an exemption from that legal requirement. The IND is the means through which the sponsor technically obtains this exemption from the FDA.
现行的联邦法律要求,一种药物在跨州运输或分销之前,必须经过上市许可申请的批准。因为临床试验申办者可能想要将临床试验用药物运送给多个州的临床研究人员,它必须寻求法律要求的豁免。IND是申办者在技术上从FDA获得这种豁免的方法。
During a new drug's early preclinical development, the sponsor's primary goal is to determine if the product is reasonably safe for initial use in humans, and if the compound exhibits pharmacological activity that justifies commercial development. When a product is identified as a viable candidate for further development, the sponsor then focuses on collecting the data and information necessary to establish that the product will not expose humans to unreasonable risks when used in limited, early-stage clinical studies.
在一种新药的早期临床前开发过程中,申办者的主要目标是确定该产品在人类初始使用中是否安全,以及该化合物是否显示出合理的商业开发药理活性。当一种产品被确定为可进一步开发的候选药品时,申办者就会集中精力收集必要的数据和信息,以确定该产品在有限的早期临床研究中使用时不会使人类面临不合理的风险。
FDA's role in the development of a new drug begins when the drug's sponsor (usually the manufacturer or potential marketer), having screened the new molecule for pharmacological activity and acute toxicity potential in animals, wants to test its diagnostic or therapeutic potential in humans. At that point, the molecule changes in legal status under the Federal Food, Drug, and Cosmetic Act and becomes a new drug subject to specific requirements of the drug regulatory system.
当药物的申办者(通常是生产商或潜在的销售商)在动物身上进行药理活性和急性毒性试验并筛选了新分子后,想要测试其在人类身上的诊断或治疗潜力时,FDA在新药开发中的作用就开始了。在这一点上,分子在联邦食品、药品和化妆品法案下的法律地位发生了变化,并成为一种受药物监管系统特定要求约束的新药。
There are three IND types
An Investigator IND is submitted by a physician who both initiates and conducts an investigation, and under whose immediate direction the investigational drug is administered or dispensed.A physician might submit a research IND to propose studying an unapproved drug, or an approved product for a new indication or in a new patient population.
研究者IND
,由发起和进行研究的医生提交,并在其直接指导下施用或分配研究药品。医生可能会提交一份研究IND,建议研究一种未经批准的药物,或一种被批准的产品,用于新的适应症或新的患者群体。
Emergency Use IND allows the FDA to authorize use of an experimental drug in an emergency situation that does not allow time for submission of an IND in accordance with 21CFR , Sec. 312.23 or Sec. 312.20. It is also used for patients who do not meet the criteria of an existing study protocol, or if an approved study protocol does not exist.
紧急使用IND
,允许FDA根据21CFR,第312.23节或第312.20节的规定,在没有时间提交IND时,授权在紧急情况下使用实验性药物。它也适用于不符合现有研究方案标准的患者,或者批准的研究方案不存在的情况。
Treatment IND is submitted for experimental drugs showing promise in clinical testing for serious or immediately life-threatening conditions while the final clinical work is conducted and the FDA review takes place.
治疗性IND
,在进行最终临床工作和FDA审查期间,提交申报在严重或立即危及生命的疾病的临床试验中有希望的临床试验药品。
There are two IND categories
Commercial
商业化
Research (non-commercial)
研究(非商业化)
The IND application must contain information in three broad areas
Animal Pharmacology and Toxicology Studies - Preclinical data to permit an assessment as to whether the product is reasonably safe for initial testing in humans. Also included are any previous experience with the drug in humans (often foreign use).
动物药理学和毒理学研究-允许评估产品是否对人类初步试验适当安全的临床前数据。还包括任何以前的人类用药经验(通常是外国使用数据)。
Manufacturing Information -Information pertaining to the composition, manufacturer, stability, and controls used for manufacturing the drug substance and the drug product. This information is assessed to ensure that the company can adequately produce and supply consistent batches of the drug.
生产信息-有关原料药和制剂的成分、生产商、稳定性和控制的信息。对这些信息进行评估,以确保公司能够充分生产和供应批间一致的药品。
Clinical Protocols and Investigator Information - Detailed protocols for proposed clinical studies to assess whether the initial-phase trials will expose subjects to unnecessary risks. Also, information on the qualifications of clinical investigators--professionals (generally physicians) who oversee the administration of the experimental compound--to assess whether they are qualified to fulfill their clinical trial duties. Finally, commitments to obtain informed consent from the research subjects, to obtain review of the study by an institutional review board (IRB), and to adhere to the investigational new drug regulations.
临床方案和研究者信息-拟定临床研究的详细方案,以评估初始阶段试验是否会使受试者面临不必要的风险。此外,关于临床研究人员资格的信息——监督实验化合物管理的专业人员(通常是医生)——以评估他们是否有资格履行其临床试验职责。最后,承诺获得研究受试者的知情同意,获得机构审查委员会(IRB)对研究的审查,并遵守临床试验用药品法规。
Once the IND is submitted, the sponsor must wait 30 calendar days before initiating any clinical trials. During this time, FDA has an opportunity to review the IND for safety to assure that research subjects will not be subjected to unreasonable risk.
一旦IND提交,申办者必须等待30个日历天才能开始任何临床试验。在此期间,FDA有机会审查IND的安全性,以确保研究受试者不会遭受不合理的风险。
This web site is designed for individuals from pharmaceutical companies, government agencies, academic institutions, private organizations, or other organizations interested in bringing a new drug to market. Each of the sections below contains information from CDER to assist you in the IND application process. For specific information, click on a link to go directly to a section or web page.
本网站是为制药公司、政府机构、学术机构、私人组织或其他有兴趣将新药推向市场的组织的个人设计的。下面的每个部分都包含CDER的信息,以帮助您进行IND。具体的信息,点击链接直接进入一个部分或网页。
Resources for IND Applications
The following resourcesinclude the legal requirements of an IND application, assistance from CDER to help you meet those requirements, and internal IND review principles, policies and procedures.
以下来自CDER的资源包括IND申请的法律要求,以满足这些法律的要求、内部IND审查原则、政策和程序。
Pre-IND Consultation Program
CDER'sPre-Investigational New Drug Application (IND) Consultation Program fosters early communications between sponsors and new drug review divisions to provide guidance on the data necessary to warrant IND submission. The review divisions are organized generally along therapeutic class.
CDER的《IND前咨询项目》可以促进申办者和新药审评部门之间的早期沟通,为保证IND提交所需的数据提供指导。一般按治疗类别咨询不同的审核组织。
Guidance Documents for INDs
Guidance documents represent the Agency's current thinking on a particular subject. These documentsprovide FDA review staff and applicants/sponsors with guidelines to the processing, content, and evaluation/approval of applications and also to the design, production, manufacturing, and testing of regulated products. They also establish policies intended to achieve consistency in the Agency's regulatory approach and establish inspection and enforcement procedures.
指导文件代表了监管机构目前对某一特定主题的想法。这些文件为FDA审查人员和申请人/申办者提供了申请的处理、内容和评估/批准指南,以及监管产品的设计、生产、制造和测试指南。它们还制定了旨在使监管机构的管理办法保持一致的政策,并制定了检查和执行程序。
Because guidances are not regulations or laws, they are not enforceable, either through administrative actions or through the courts. An alternative approach may be used ifit satisfies the requirements of the applicable statute, regulations, or both. For information on a specific guidance document, please contact the originating office.
由于指导方针不是法规或法律,因此无论是通过行政行为还是通过法院,它们都不能强制执行。如果替代方法满足适用的法规、规章或两者的要求,则可以使用替代方法。有关具体指导文件的信息,请与文件对应的办公室联系。
To find guidance documents to help prepare INDs,go to Guidances (Drugs) and use "investigational" in the search box.
要查找INDs的指导文件,请访问“Guidances (Drugs)”,并在搜索框中使用“investigational”。
Laws, Regulations, Policies and Procedures
The mission of FDA is to enforce laws enacted by the U.S. Congress and regulations established by the Agency to protect the consumer's health, safety, and pocketbook. The Federal Food, Drug, and Cosmetic Act is the basic food and drug law of the U.S. The law is intended to assure consumers that foods are pure and wholesome, safe to eat, and produced under sanitary conditions; that drugs and devices are safe and effective for their intended uses; that cosmetics are safe and made from appropriate ingredients; and that all labeling and packaging is truthful, informative, and not deceptive.
FDA的使命是执行美国国会制定的法律和该机构制定的法规,以保护消费者的健康、安全和钱包。《联邦食品、药品和化妆品法》是美国的基本食品和药品法,该法旨在向消费者保证食品是纯净、健康、可安全食用的,并在卫生条件下生产;药品和器械对其预期用途是安全有效的;化妆品是安全的,由合适的成分制成;所有的标签和包装都是真实的、信息齐全的、没有欺骗性的。
Code of Federal Regulations (CFR)
The final regulations published in the Federal Register (daily published record of proposed rules, final rules, meeting notices, etc.) are collected in the Code Of Federal Regulations (CFR). The CFR is divided into 50 titles that represent broad areas subject to Federal regulations. The FDA's portion of the CFR interprets the The Federal Food, Drug, and Cosmetic Act and related statutes. Section 21 of the CFR contains most regulations pertaining to food and drugs. The regulations document all actions of all drug sponsors that are required under Federal law.
在《联邦公报》上公布的最终法规(建议规则、最终规则、会议通知等每日公布的记录)收集在《联邦法规法典》(CFR)中。CFR分为50个标题,代表受联邦法规约束的广泛领域。FDA的CFR部分解释了联邦食品、药品和化妆品法案及相关法规。CFR第21节包含大多数与食品和药品有关的法规。这些法规记录了所有药品发起者在联邦法律要求下的所有行动。
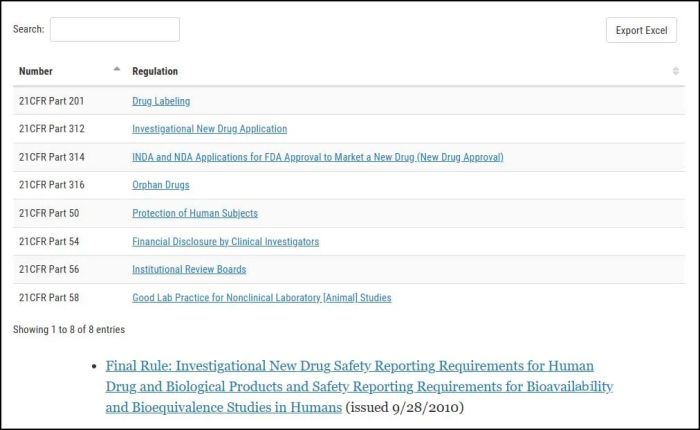
The following regulations apply to the IND application process:
下列规定适用于IND申请程序:
Manual of Policies and Procedures (MaPPs)
CDER's Manual of Policies and Procedures (MaPPs) are approved instructions for internal practices and procedures followed by CDER staff to help standardize the new drug review process and other activities. All MAPPs are available for the public to review for a better understanding of office policies, definitions, staff responsibilities and procedures. To find MaPPs of particular interest to IND sponsors, go to CDER Manual of Policies and Procedures and use "INDs" in the search box.
CDER的政策和程序手册(MaPP)是CDER员工遵循的内部实践和程序的批准指南,以帮助标准化新药审查过程和其他活动。所有MaPP均可供公众查阅,以便更好地了解办公室政策、定义、员工职责和程序。要查找IND发起者特别感兴趣的MaPP,请访问CDER政策和程序手册,并在搜索框中使用“INDs”。
Emergency Use of an Investigational Drug or Biologic
Emergency Use of an Investigational Drug or Biologic - Information Sheet.
TheGuidance for Institutional Review Boards and Clinical Investigators contains information on: Obtaining an Emergency IND, Emergency Exemption from Prospective IRB, Approval Exception from Informed Consent, and Requirement Planned Emergency Research, Informed Consent Exception.
《机构审查委员会和临床研究者指南》包含以下信息:获得紧急IND、预期IRB的紧急豁免、知情同意的批准例外、要求计划的紧急研究、知情同意例外。
Physician Request for a Single Patient IND for Compassionate or Emergency Use.
Instructions for Sponsors of Emergency Investigational New Drug (EIND) Applications for Antimicrobial Products. From the Office of Antimicrobial Products, Division of Antiviral Products.
抗微生物药物紧急研究新药(EIND)申请的申办者须知。来自抗病毒产品部抗菌产品办公室。
For investigational biological products regulated by CBER, call800-835-4709 or 240-402-8020.
对于CBER管理的临床研究用生物制品,拨打800-835-4709或240-402-8020。
For all other investigational drugs, call301-796-3400.
对于其他临床试验用药品,拨打301-796-3400。
After working hours, call FDA’s Office of Emergency Operations at1-866-300-4374 or 301-796-8240.
非工作时间,拨打FDA紧急运营办公室1-866-300-4374或301-796-8240。
撰稿人 | GMP干货
责任编辑 | 胡静
审核人 | 何发















评论
加载更多